By using this website, you agree to our Privacy Policy
×
What is Vulcanisation?
Creaing a Stable Product
The process of creating a stable product using heat, sulphur and other means.
Rubber only became truly useful after the discovery of vulcanisation. Vulcanisation originally referred to the process of heating rubber with sulphur to create a stable product not susceptible to changes in temperature.
The transformation from a material with thermoplastic properties to a thermoset, an elastomer. It now, along with the term cure, refers to any method used to do this.
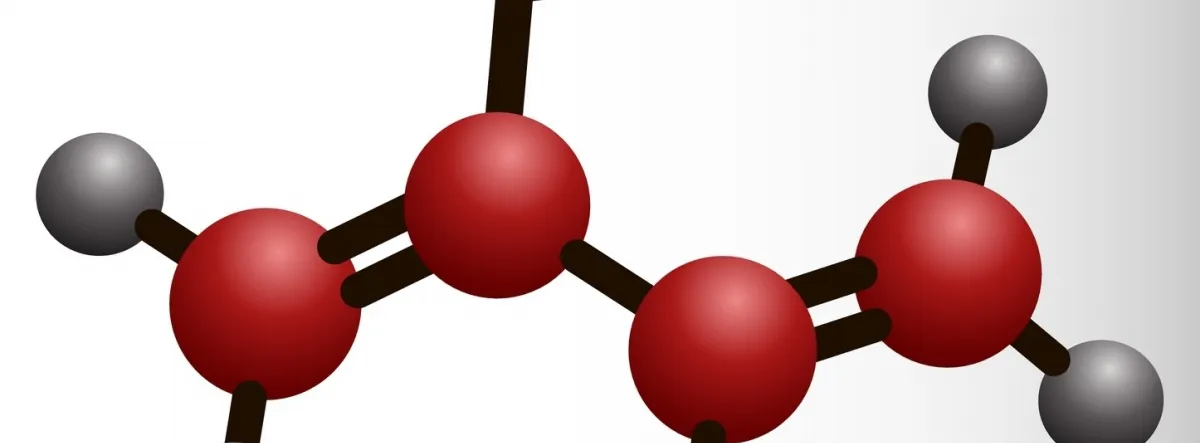
During sulphur vulcanisation, sulphur cross-links are formed between carbon atoms in different chains. The links are formed between reactive sites in the carbon chain called double bonds. Heat is required to start the reaction between the carbon atoms and the sulphur.
Once the crosslinks are formed, the long crumpled chains can be pulled apart, but on the release of the stretching force, the sulphur links ensure they return to their original conformation.
The sulphur links can range from one sulphur atom to several depending on the amount of sulphur and other additives used. This number affects the properties of the product.
After the Vulcanisation Reaction
Once the vulcanisation reaction has happened, the rubber product recovers almost fully after deformation. This is in complete contrast to plastics which melt when heated and permanently lose their shape.
However, plastics can be reformed multiple times, whereas once the vulcanisation reaction has happened, the rubber cannot be reprocessed; it has “set”.
The original reaction time to form a vulcanised rubber article was about 8 hours. Following experimentation, it was very quickly discovered that the addition of metal oxides reduced reaction time to 4 hours.
In the early 20th century, organic accelerators were identified that reduced the reaction time to an economic number of minutes.
Crosslinking is the general term for the process of forming bonds or relatively short sequences of chemical bonds to join two polymer chains together. In addition to reaction with sulphur, a number of methods have been developed to do this, driven by the particular needs of a given polymer and the end properties required from the cross-linked polymer.
Cross-Linking Unsaturated Polymers
Peroxides are useful in cross-linking unsaturated polymers where certain properties are required.
Peroxides are used for crosslinking saturated polymers where there is no reactive double bond, for example, EPDM.
However, they are also useful in cross-linking unsaturated polymers where certain properties are required.
Instead of sulphur links between the polymer chains, carbon links are formed by free radical reactions following the decomposition of the peroxide.
Advantages
Improves compression set at elevated temperatures.
Improves high-temperature stability.
Enables a sulphur-free compound necessary to prevent copper corrosion.
Disadvantages
Lower tensile and tear strength.
Lower abrasion resistance.
Reduced swell resistance.
Some polymers cannot be cross-linked using sulphur, for example, chloroprene.
The chlorine atoms attached to the carbon backbone sterically hinder any reaction at the double bonds. A combination of Zinc and magnesium oxides has been found to be effective in enabling the formation of carbon cross-links.
As each polymer has a different chemical formula, each has a number of specific combinations of metal oxides, sulphur, sulphur donor activators and organic accelerators etc., identified as being the most efficient at achieving a practical state of cure with the desired properties.
The links produced between the polymer chains are quite different depending on the vulcanisation system used.
We’ll Get Straight Back to You
Explore our other Industries
Speak to One of Our Experts
Contact UsLEARN
INDUSTRIES
PRODUCTS
BRANDS
GET IN TOUCH


- © 2026 Nufox. All rights reserved |
- Terms & Conditions |
- Privacy Policy |
- Download ISO Certificate | Web Design MadeByShape